CMS Regulated
CMS Regulated is very similar to standard CMS. The main difference is in the implementation and available modules.
The expectation of CMS Regulated customers is that they will validate the system. That validation would be to FDA 21 CFR Part 11 standards for electronic signatures. That is to say, in a manual operation, there must be two (2) signers for every key document. When CMS is validated to 21 CFR Part 11 standards, the software is the first checker and the user is the second checker. Since the system is paperless, they operator must sign the document electronically.
This has a tremendous impact on ROI calculations as users can work autonomously, without the need for a second user or manager to be nearby.
The modules included in CMS Regulated that are not in CMS regular are electronic signatures and resource maintenance.
The implementation of CMS Regulated is also different. When installing in a regulated environment, the end user gets 3 databases. One for development and rough testing, another for applying scripts and testing those scripts and lastly a live database that is strictly secured from any modifications.
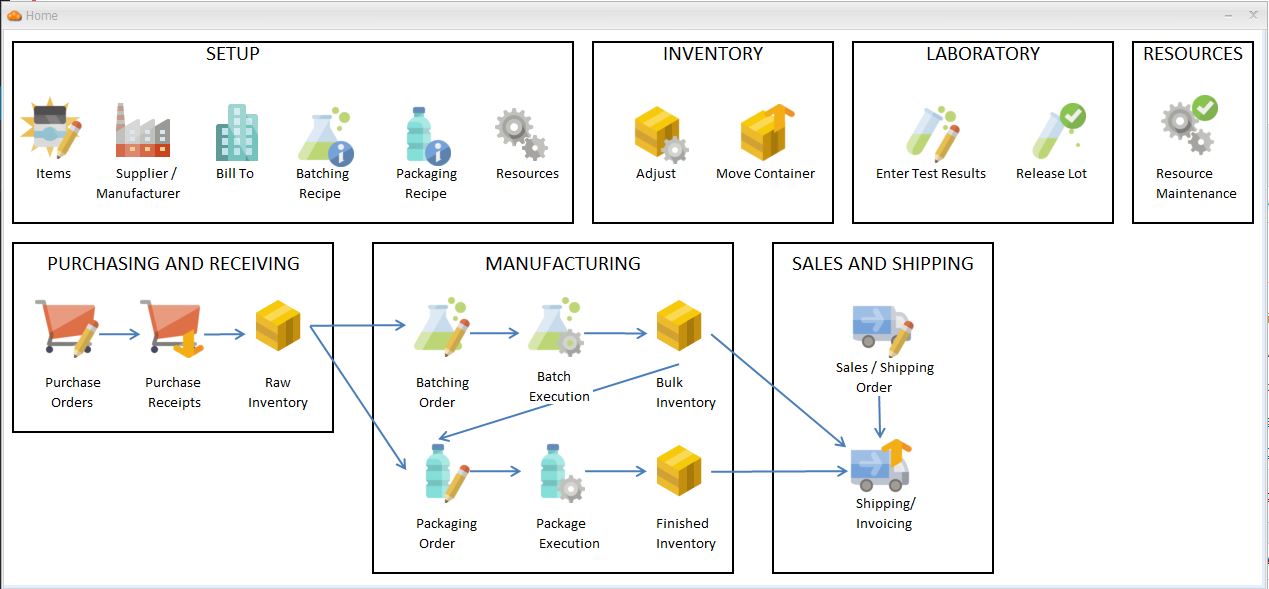

Laboratory Information Management

Purchasing / Sales Orders

Host Connectivity

Recipe Development

Manufacturing Execution

Resource Maintenance

Material Resource Planning

Barcode Warehousing
